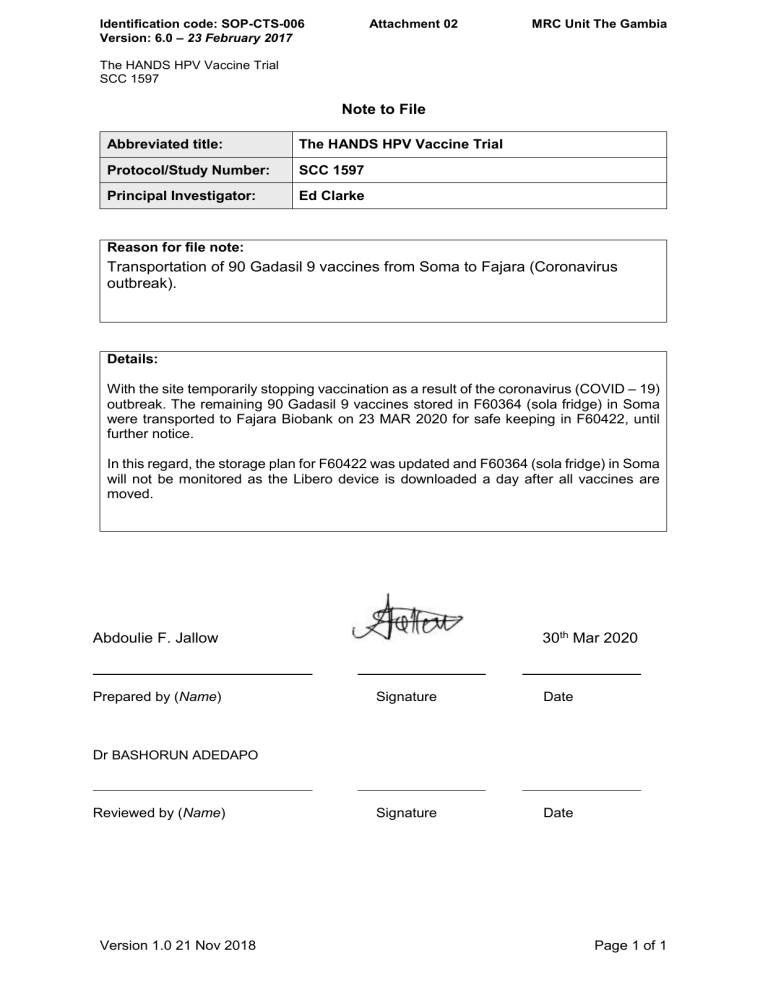
Note To File Template - Web 2.1 all notes to the study file should be signed by the author, kept on file in the site regulatory file and made available to the clinical site monitors reviewing the site’s. A descriptive narrative offering advice. Web click the icon that reads import on the play screen. Web a note to file should: The templates are optional and. Web find templates and guidelines for clinical research study protocol, data safety monitoring plan, data sharing plan, and more. Web study teams should review all available tools and what will be needed for compliant and complete documentation. This web page provides a description. These templates are designed to help meet requirements for. Web this template can be used to document various events or issues that occur throughout the course of a research study.
NoteToFile Template Instructional Doc Template pdfFiller
Web if the issue relates to site performance, the appropriate credentialed individual from the site should write and sign the note to file. Web part.
Note To File Sample PDF Template
A useful ntf has the following parts: Web if the issue relates to site performance, the appropriate credentialed individual from the site should write and.
Legal File Note Template Sample Design Layout Templates
Patients xxx through xxx all signed informed consent prior to any. Web find templates for clinical research protocols, informed consent materials, regulatory documents, and case.
NoteToFile Template
Web find three examples of note to file templates in doc format for research purposes. Patients xxx through xxx all signed informed consent prior to.
Note to File Template Doc Template pdfFiller
Web you might just need to refresh it. A descriptive narrative offering advice. The templates are optional and. From the minecraft education start screen, click.
Note To File Sample PDF Template
Web learn how to avoid unnecessary and inappropriate use of ntfs in clinical trials, and how to write a complete and accurate ntf when needed..
Note To File Template Download by Pharma Student Issuu
Web find templates for clinical research protocols, informed consent materials, regulatory documents, and case report forms on this web page. Web find templates and guidelines.
Meeting Notes Template 30+ Word, PDF Documents Download
A descriptive narrative offering advice. Web study teams should review all available tools and what will be needed for compliant and complete documentation. It is.
181121 File Note template
When used properly, an ntf can be a positive practice. Web note to file template to be used to create a note to file which.
Web 2.1 All Notes To The Study File Should Be Signed By The Author, Kept On File In The Site Regulatory File And Made Available To The Clinical Site Monitors Reviewing The Site’s.
Web visiting the administrator hub area to locate administrator guidance and release notes. These templates are designed to help meet requirements for. The templates are optional and. A descriptive narrative offering advice.
Note To File Is A Record Of Events, Actions, Or Decisions Related To A Research Project Or Activity.
The problem is that choosing “save as…” or “save a copy. Web study teams should review all available tools and what will be needed for compliant and complete documentation. Web find templates and guidelines for clinical research study protocol, data safety monitoring plan, data sharing plan, and more. Ithenticate and crossref similarity check guidance is now located on a separate site.
Please Note That This Page Has Been Updated For 2015 Following A Quality Check And Review Of The Templates, And.
Be signed and dated by the individual who is writing it. Find the.mcworld file and select it to import. Web ðï ࡱ á> þÿ. A useful ntf has the following parts:
Web Find Three Examples Of Note To File Templates In Doc Format For Research Purposes.
Patients xxx through xxx all signed informed consent prior to any. It is used to clarify an error, omission or discrepancy or to document a problem or corrective action. Web you might just need to refresh it. Web a note to file should: